WARNING: This product contains nicotine.
Nicotine is an addictive chemical.
Nicotine is an addictive chemical.
Apr 8,2021

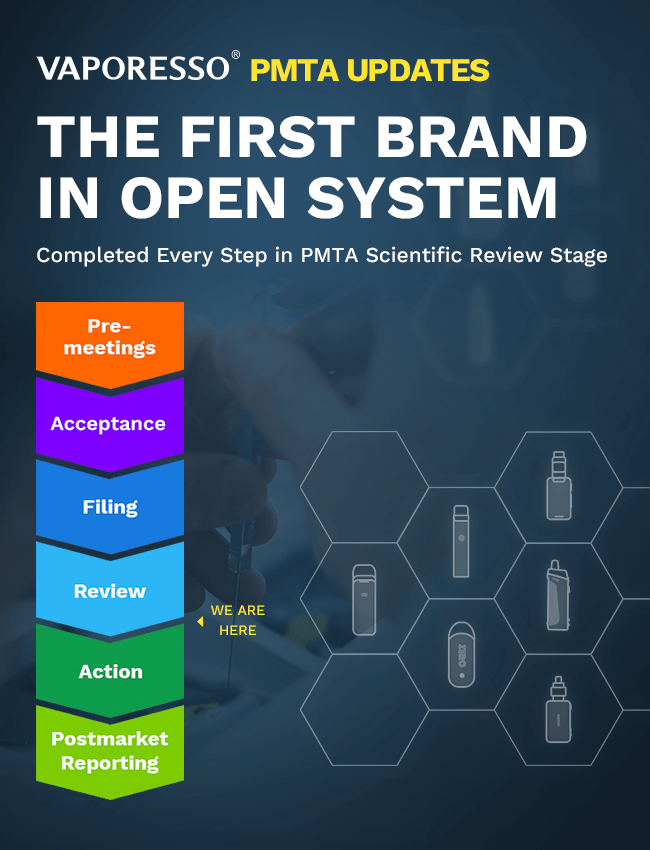
On Mar 12, 2021, Vaporesso responded to the FDA
deficiency letter.
On Apr 8, 2021, Vaporesso has completed the FDA Remote
Regulatory Assessments
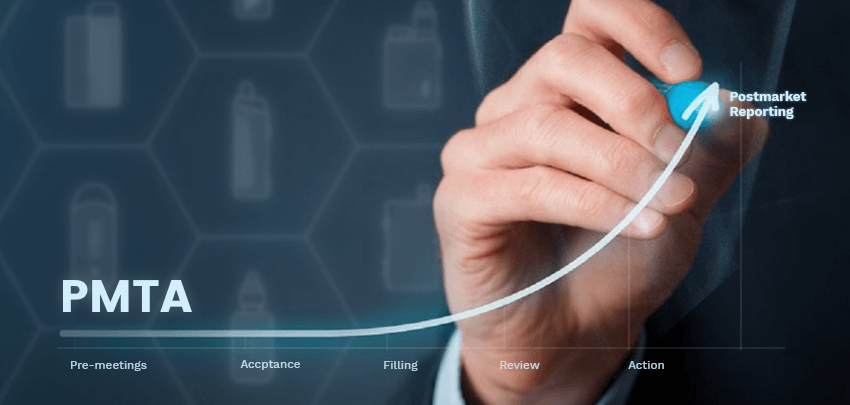
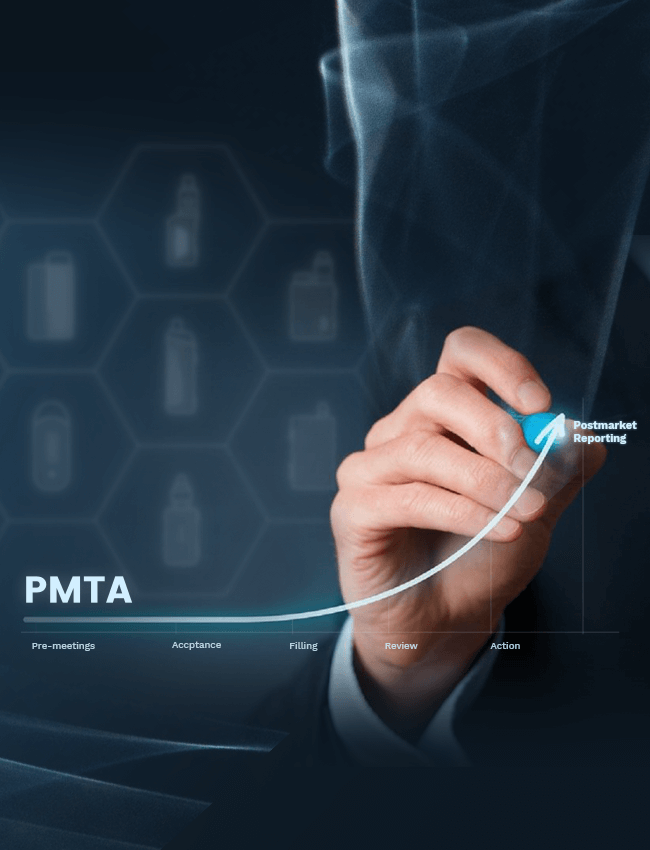
Now Vaporesso has completed all the process in the
scientific review stage and waiting for the final
decision from FDA.
Sep 1, 2020


First group of PMTA products submitted by VAPORESSO,
SMOORE's self-owned brand, has passed the FDA's second
round - the filing stage.
Aug 20, 2020
On August 20, 2020, VAPORESSO received the acceptance
letter for the first round of its PMTA applications
from the FDA - only three days after submission.
According to its U.S. scientific CRO agent, the
application received positive comments from FDA on its
overall preparation.
Learn more >

Get the latest product launches, promotions, and contests delivered straight to your inbox for free! Remark: To improve our service quality, by submission you accept the third party's survey assigned by Vaporesso about your comments on our products
 marketing@vaporesso.com
marketing@vaporesso.com
 support@vaporesso.com
View More...
support@vaporesso.com
View More...
 +86 18925236359
+86 18925236359
 anticf@smoorecig.com
anticf@smoorecig.com
Vaporesso e-cigarette devices are intended for use with
e-liquids, which may contain nicotine. Nicotine is an
addictive chemical. Do not use with any other
substances. Do not get on skin or in eyes. Do not drink.
Store in original container and keep away from children
and pets. In case of accidental ingestion, call the
Poison Control Center at 1-800-222-1222.
This product is intended for adult users of
nicotine-containing products, particularly current
smokers or vapers. Underage sale is prohibited. Do not
use this product if you:
• Are under the legal age of purchase
• Are pregnant or breastfeeding
• Have heart disease, stomach or duodenal ulcers, liver
or kidney problems, throat disease, or difficulty
breathing due to bronchitis, emphysema, or asthma
• Have an overactive thyroid or pheochromocytoma (a
tumor of the adrenal gland that can affect blood
pressure)
• Are taking certain medications, such as theophylline,
ropinirole, or clozapine
CALIFORNIA PROPOSITION 65 - Warning: This product can
expose you to chemicals including formaldehyde and
acetaldehyde which known to the State of California to
cause cancer, and Nicotine, which is known to the State
of California to cause birth defects or other
reproductive harm. For more information go to
www.P65Warnings.ca.gov
Copyright © 2022 Vaporesso. All rights reserved. Privacy | Terms & Conditions | Cookies policy
